Description
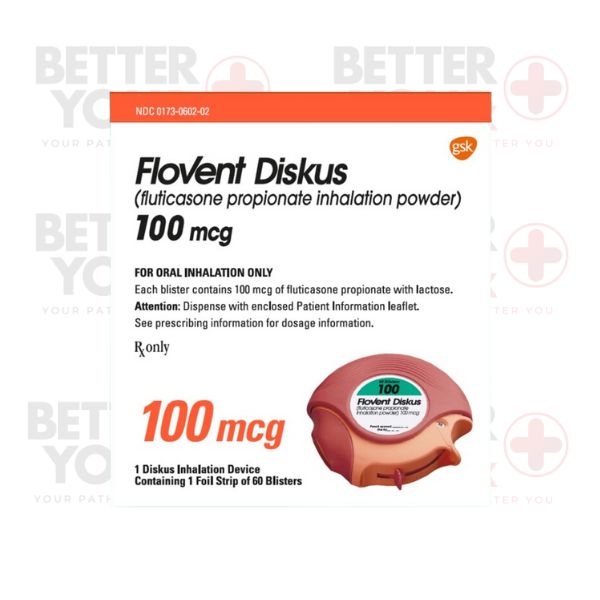
Flovent Diskus falls under the category of inhaled corticosteroids (ICS), pivotal for long-term asthma management, aiming to alleviate airway inflammation and forestall asthma symptoms.
The principal role of Flovent Diskus lies in controlling and preventing asthma symptoms, encompassing wheezing, breathlessness, and coughing. By diminishing airway inflammation, it fosters enhanced asthma management and an overall improved quality of life. Parallel agents addressing asthma and/or COPD include Breo and Trelegy.
The pivotal component within Flovent Diskus is fluticasone propionate. Upon inhalation, this corticosteroid targets specific receptors within the airways, curtailing the generation of inflammatory agents and averting airway constriction.