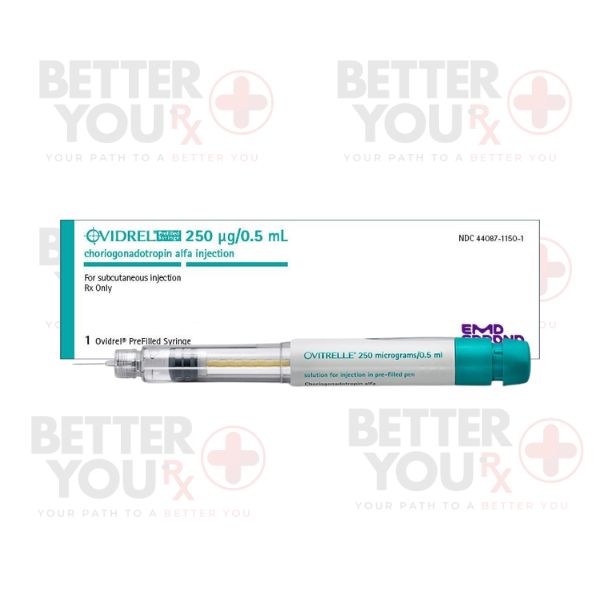
Indications:
●Inducing ovulation in women undergoing fertility treatments
●Preparing the ovaries for egg retrieval in procedures such as IVF
●Sometimes used in conjugation with other fertility treatments to enhance ovulation efficiency
Administration Guidelines
●Ovidrel Syringe is administered as a subcutaneous injection, usually into the abdomen or thigh.
●The healthcare provider will specify the exact timing for the injection, typically 24 to 36 hours before the scheduled ovulation or egg retrieval procedure.
●Patients are advised to follow the instructions provided by their healthcare provider or the medication guide that comes with the syringe.
●Before use, inspect the syringe for any particulate matter or discoloration. Do not use if the solution is cloudy, discolored, or contains particles.
●Gently pinch the skin where the injection is to be administered, insert the needle at a slight angle, and slowly press the plunger to inject the medication.
Administration Details
Dosage Forms:
●Prefilled syringe containing recombinant hCG
Dosage:
●The standard dosage is usually one injection of the dose prescribed by the healthcare provider, based on individual treatment plans and response to therapy.
Safety Precautions
●Pregnancy and Lactation: Use Ovidrel only under the direct guidance of a fertility specialist. Discuss potential risks and benefits with your healthcare provider before starting treatment.
●Ovarian Hyperstimulation Syndrome (OHSS): Women undergoing fertility treatments with Ovidrel are at risk for developing OHSS, a condition characterized by enlarged ovaries and fluid accumulation in the abdomen. It's crucial to monitor for symptoms and report any severe abdominal pain, nausea, or swelling immediately.
Drug Interactions
●Inform your healthcare provider about all current medications and supplements. While specific interactions with Ovidrel are not well-documented, it's important to consider potential interactions with other fertility drugs.
|



Reviews
There are no reviews yet.