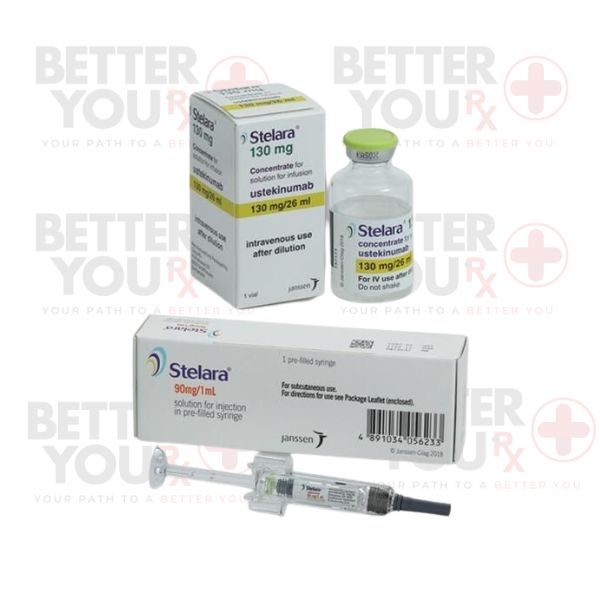
Description
Stelara, a remarkable monoclonal antibody, falls under the category of interleukin (IL) inhibitors—a class of medicines that wields transformative power against autoimmune ailments. Elevated levels of interleukin-12 (IL-12) and interleukin-23 (IL-23) proteins are often associated with autoimmune disorders. Stelara steps in by thwarting these proteins, leading to decreased inflammation, pain, swelling, and skin-related symptoms.
Having received Federal Health Authority approval on September 25, 2009, Stelara initially targeted moderate to severe psoriasis in adults. Its efficacy has since extended to multiple conditions:
- Psoriasis: Offering a reprieve from the torment of itchy, red, and scaly skin patches, Stelara redefines the management of chronic psoriasis by addressing its root cause—immune dysregulation.
- Psoriatic Arthritis: For those battling joint pain and stiffness in tandem with psoriasis, Stelara’s unique approach brings relief to both skin and joints, significantly enhancing quality of life.
- Crohn’s Disease: Stelara’s impact reaches the digestive realm, as it tames the overactive immune response in Crohn’s disease, aiding in healing and inflammation reduction.
- Ulcerative Colitis: Alleviating ulcerative colitis—marked by colon and rectum inflammation and ulcers—Stelara’s precision immune modulation is a powerful tool against this inflammatory bowel disease.
To address Crohn’s disease and ulcerative colitis, Stelara employs an intravenous (IV) infusion, followed by subsequent subcutaneous injections. These injections, whether self-administered or by a healthcare professional, continue to redefine treatment outcomes.





Reviews
There are no reviews yet.